By CAFMI AI From Nature Reviews Immunology
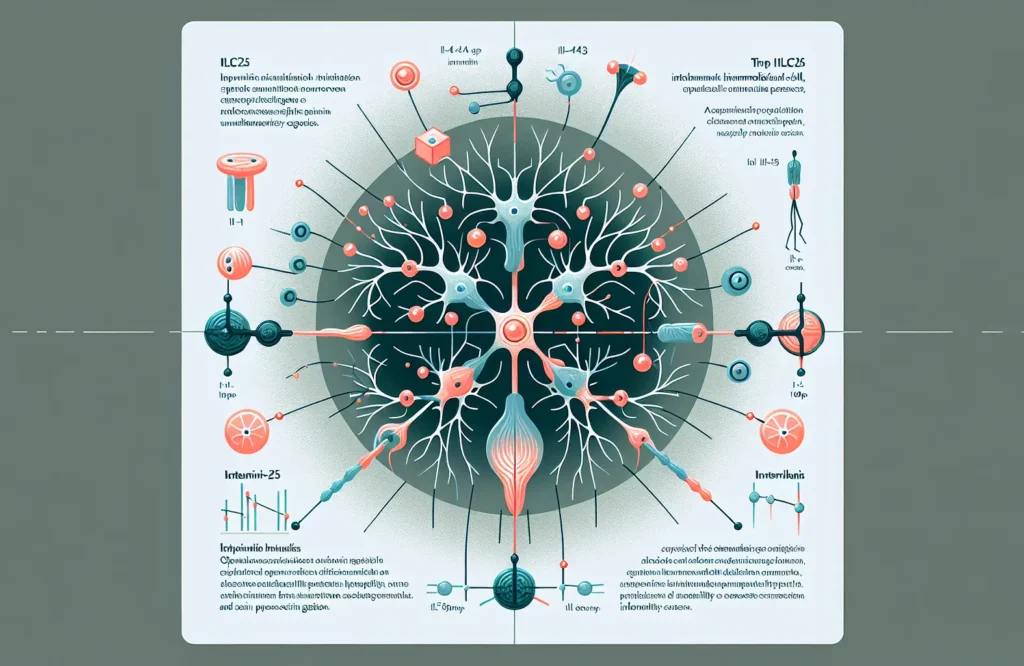
The interaction between type 2 innate lymphoid cells (ILC2s) and neurons represents a significant advancement in understanding pain modulation and its clinical implications. ILC2s, a subset of immune cells traditionally associated with responses to parasites and allergens, have been found to interact directly with sensory neurons located in peripheral tissues. This communication is facilitated through ILC2 secretion of cytokines such as interleukin-5 (IL-5) and interleukin-13 (IL-13), which influence neuronal excitability and pain signal processing. The cross-talk between ILC2s and nociceptive neurons modulates pain sensitivity by either amplifying or dampening nociceptive signals depending on the inflammatory context. For clinicians, recognizing this neuro-immune interaction provides insight into how immune dysregulation might contribute to chronic pain syndromes, especially those resistant to traditional analgesics. These findings also suggest that immune cell activity is integral not only to inflammation but also to sensory perception, potentially explaining variations in pain responses among patients with inflammatory diseases.
This new understanding of ILC2-neuron interactions carries significant clinical repercussions. The identification of cytokines, particularly IL-5 and IL-13, as mediators that alter neuronal excitability opens avenues for therapies targeting these molecular pathways to modulate pain. From a clinical standpoint, such immune-targeted treatments could complement existing analgesics and offer alternatives for patients suffering from chronic pain conditions and immune-related gait abnormalities. Moreover, the nuanced regulation of pain by ILC2s suggests that therapeutic interventions could be personalized according to patients’ inflammatory profiles, improving efficacy while minimizing side effects. Clinicians should also consider that immune modulation may not only alleviate pain but also impact locomotor functions, as changes in pain perception and gait are interconnected. Understanding these mechanisms encourages integrative patient management approaches involving pain specialists, immunologists, and rehabilitation experts to optimize outcomes.
While these insights are promising, it is important to consider the limitations and areas for future research. Most current data derive from animal models and in vitro studies, which, although informative, require further validation in human clinical settings to confirm applicability. Population heterogeneity and comorbid conditions in clinical environments may influence the ILC2-neuron cross-talk differently, necessitating well-designed clinical trials. Moreover, the precise signaling pathways and the balance between cytokine-mediated excitation and inhibition of neurons need clarification to avoid unintended consequences of immune-targeted therapies. From a practical perspective, clinicians should also be aware of red flags that suggest underlying immune or neurological disorders when evaluating pain and gait abnormalities. Patient counseling should include education on the potential immune links to sensory symptoms and motor function changes. Follow-up should involve multidisciplinary evaluations to monitor pain control, immune activity, and gait progression. Overall, integrating neuro-immune perspectives into primary care workflows could enhance diagnostic accuracy and treatment efficacy for complex pain syndromes.
Read The Original Publication Here